Expedited Review
IRB Forms
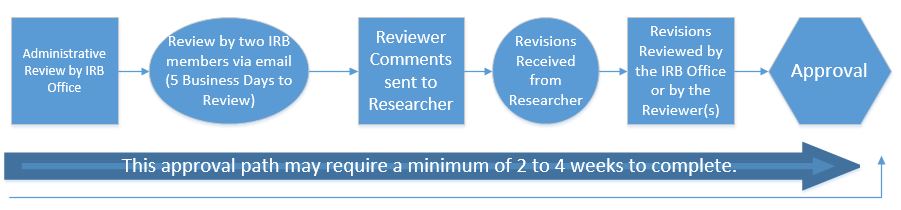
Expedited Review Process
For human subjects research involving no more than minimal risk*, and for minor changes or amendments to approved research.** Expedited review categories are specified in the regulation that governs the Institutional Review Board (45 CFR 46.110). Categories 5, 6 and 7 are most commonly used at GS.
*It cannot be assumed that research poses minimal risk because it involves only interview or survey data collection. Sensitive questions may lead to distress that exposes participants to greater than minimal risk. Loss of confidentiality can cause harm to participants, their relatives, and others. (The same forms are used to apply for expedited or full board review.)
**This process may also be used for minor changes to research approved by the full board process at the IRBs discretion.
Forms are updated regularly to capture changes in regulatory requirements. Use current forms from the website for all submissions.
Expedited Forms and Resources |
|
|
|
| Form Name | Additional Form Information |
| IRB Application | Complete for all expedited and full board reviews. Note: Do not include dissertation or thesis chapters. Information required for review must be condensed into the application format. REQUIRED FOR ALL EXPEDITED AND FULL BOARD |
| COVID Safety Plan |
COVID Safety Plans for in-person research are now embedded directly in the IRB application and informed consent. The language represents the minimum requirement. Researchers must still follow more stringent protocols if required by the research location, local conditions or participant request. |
| CITI Human Subjects Training |
CITI Program training provides social/behavioral content training. Online certificate will be provided to you once the course is completed. Attach certificate or transcript to each protocol submitted. Certificates are not maintained by the IRB. Training certificates from previous submissions are not readily available. Click here for instructions. REQUIRED EACH TIME YOU APPLY. |
|
(Consent format not required.) |
This is a sample of an informed consent document provided on an electronic version of GS letterhead. Any information in the sample should be edited as appropriate for the population you are using and the type of research you are conducting. Click here for further information on HIPAA consent criteria. |
| Informed Consent Checklist | Tool for the construction of an Informed Consent document. – Required content |
|
(Assent format not required.) |
This is a sample of a minor assent document provided on an electronic version of GS letterhead. Any information in the sample should be edited as appropriate for the population you are using and the type of research you are conducting. Your assent should be on a reading level appropriate for the participant’s age. |
| Parental Informed Consent Sample (Consent format not required.) | Parental consent can follow the same guide as the general consent but should be addressed to the parent and focus on the risk and benefits to their children. |
| Instrument | Please include a copy of all instruments to be used in the research (surveys, questions, etc.) |
| Recruitment Materials | Please include a copy of all materials to be used for recruitment (flyers, emails, etc.) |
|
Sample Letter of Cooperation (LOC) or Site Authorization (Letter of cooperation format not required.) |
If subjects are students or employees of another institution, include a written letter of cooperation from the subjects. This may be a letter or email from the institutional official’s professional address with the institutional officer and title identified in the email body. All letters of cooperation must clearly identify the investigator and project by title. (See the FAQ for additional information.) Note: If using education data obtained from school records you must include documentation of parental permission for the release of such data under the Family Educational Privacy Act (FERPA). This may be a copy of the release form, informed consent, or an assurance from the institutional official that such permission has been obtained prior to data release. |
|
Research Participant Opportunities page request form |
The Research Services office supports the Research Participant Opportunities page housed on the my.georgiasouthern.edu portal. Studies advertised on this page are available from the Research Tile continuously. The page is highlighted on the my.messages tile when new studies are added. To add your IRB approved study, complete the request form. |
| Policies | These are policies that often apply to projects |
| Georgia Southern Survey Policy | University policy for the use of the GS email system for distribution of surveys to faculty, staff, and students. |
| Human Subject Incentive Payment Policy and Procedure – FS-AP-1304-01 | This is a Finance and Business Operations Policy. Incentives control log can be found on the finance page under other forms. |
| Security Standards for Information Systems – IT-3610-00 | IT policy – data security requirements |
| Data Stewardship and Classification Standard | IT policy – Class I – Confidential; Class II – Sensitive with protection defined |
Last updated: 3/21/2023
